INFORMATION BELOW IS FOR THE RESEARCH & DEVELOPMENT OF DUAL-ANTIBAX
DUAL-ANTIBAX: COMPACT POWDER FOR ANTIBACTERIAL WOUND HEALING AGENT
Brief Summary
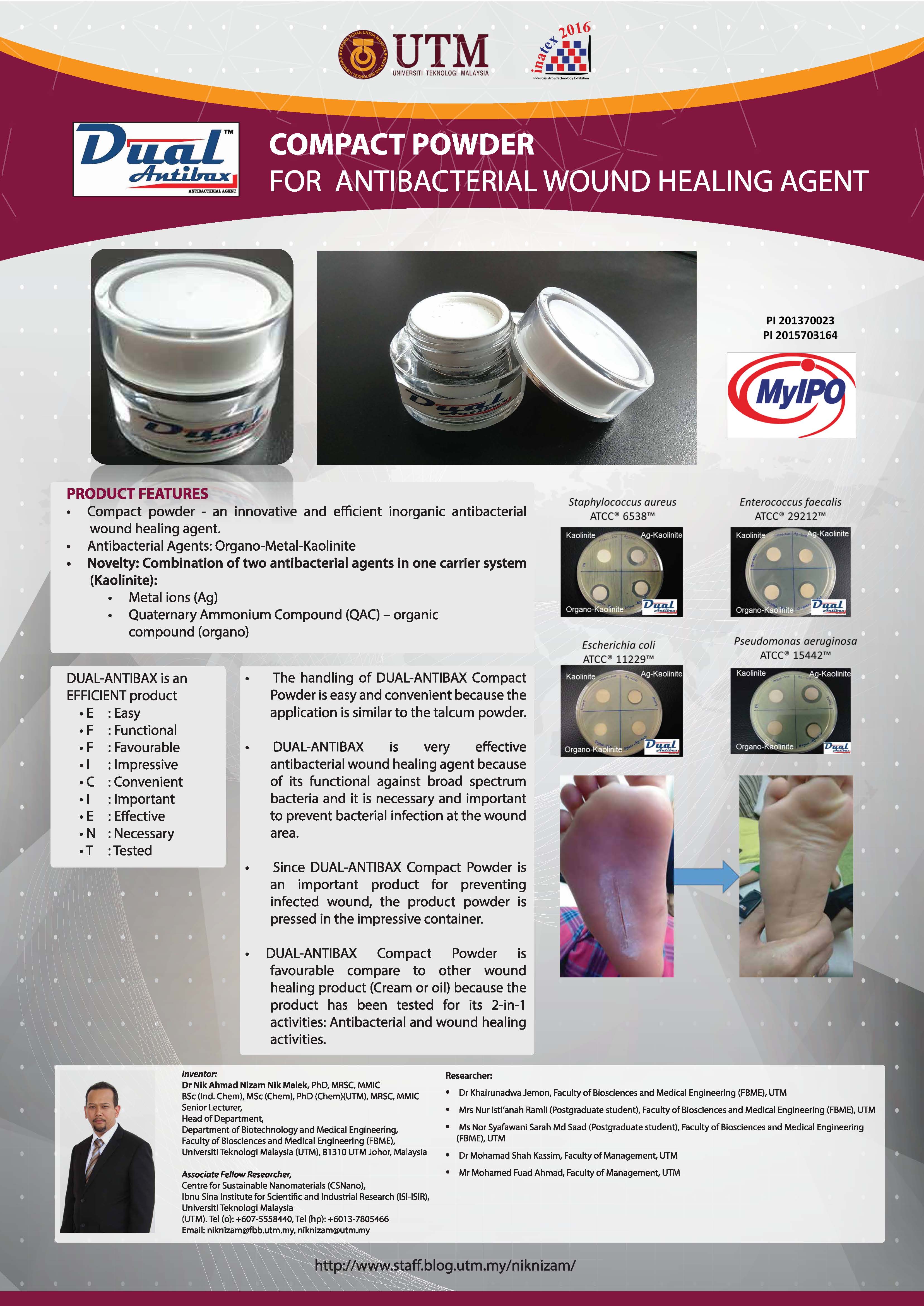
DUAL-ANTIBAX® is an innovative inorganic antibacterial agent, a material that can kill pathogenic bacteria. The product is in fine powder form and insoluble in water. The main ingredients of DUAL-ANTIBAX® are kaolinite as a major ingredient and silver ions and cetyltrimethyl ammonium bromide (CTAB) as its active ingredients. The process of making DUAL-ANTIBAX® (Patent Filing No: PI 201370023) and the application of DUAL-ANTIBAX® as antimicrobial wound healing agent (Patent filing No: PI 2015703164) were filed for patent under Universiti Teknologi Malaysia.
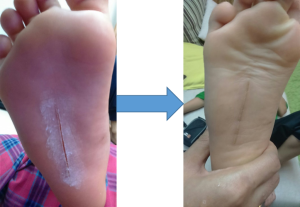
DUAL-ANTIBAX® compact powder is a product for treating wound and preventing bacterial infection at the wound area. This product has been proven to be safe to human skin based on our cytotoxicity test (Report on in vitro cytotoxicity and wound healing activity of DUAL-ANTIBAX® by IBD, UTM) and primary skin irritation test (Report No: MB-035-3714-PSI-01-10). Our targeted customers are pharmacies, hospitals and clinics and out targeted patients are those who are suffering with diabetic or person with underlying immune system problems that can easily develop infected wound on their skin. Furthermore, in other application, this product can also be mixed in the cosmetic products to prolong their shelf-life by preventing contamination to the product. Targeted market for this product and purpose is local and international cosmetic manufactures.
The research and development of this product has been funded by several research grants worth of RM 250 000.00. Now, it is ready for pre-commercialization stage. A conclusion from business model and market study report prepared by Innovation and Commercialisation Centre (ICC), Universiti Teknologi Malaysia quotes:
“The product i.e. DUAL-ANTIBAX demonstrates great potential of succeeding in the market and with the expertise of the inventor the probability of success is greatly enhanced.”
PRODUCT FEATURE
| Product name | DUAL-ANTIBAX® Compact Powder |
|
Application
|
Antibacterial wound healing agent |
| Need |
Wound can be easily infected by bacteria and once infected; the healing process will be delayed. The infected wound on human skin usually occurs for the person who has low immune system and also diabetic patients. Infections usually require proper wound care time, expensive drugs and antibacterial therapies and may increase morbidity. These socioeconomic impacts on wound management certainly emphasize the need for acceleration of wound healing process, reduce scarring and improve appearance of the healed wound. |
| Approach |
DUAL-ANTIBAX®: Two antibacterial agents in one carrier system could be used to treat wound to prevent bacterial infection. Besides its dual antibacterial agent, DUAL-ANTIBAX® also has dual activities: antibacterial and wound healer. Additionally, its antibacterial activities is for wide spectrum of pathogenic bacteria. |
| Benefits |
Inside DUAL-ANTIBAX®, a kaolinite carries low amounts of silver ions and CTAB (cetyltrimethyl ammonium ions). Unlike other products, DUAL-ANTIBAX can deliver these two antibacterial agents at the wound site and prevent bacteria to grow or kill the bacteria cells. Furthermore, it can prevent the release of vast amount of silver ions and particles into environment. |
| Competition |
The application of DUAL-ANTIBAX in wound management will be competed with other existing wound healing agent in the market such as antibiotic cream, silver sulfadiazine, etc. However, this product provide alternative to the existing wound healing agent. The most important of this product is its dual functions: antibacterial and wound healing activities. Furthermore, it is for wide spectrum of bacteria unlike most of the antibiotic cream. |
PRODUCT FACTS
| ITEMS | DOCUMENTATION |
| Patents |
PI 201370023, Antibacterial agent (04 Jan 2013) PI 2015703164, Antimicrobial Wound Healing Agent (11 Sep 2015) |
| Trademark Logo | Registration No: 2014 065397 (Class 1), 2014 065407 (Class 2), 2014 065409 (Class 5). (13 Oct 2014) |
| Wound Healing Activity and Cytotoxicity (in vitro) | Report on in vitro cytotoxicity and wound healing activity on DUAL-ANTIBAX prepared by Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia. (12 Feb 2015) |
| Wound Healing Activity (in vivo) | Report No. MB-035-3714-WVIV-01-01, Investigation of Wound Healing Activity by Makmal Bioserasi, Centre for Research and Instrumentation Management (CRIM), Universiti Kebangsaan Malaysia. (30 Dec 2015) |
| Primary Skin Irritation Test (in vivo) | Report No. MB-035-3714-PSI-01-10, Primary Skin Irritation Test by Makmal Bioserasi, Centre for Research and Instrumentation Management (CRIM), Universiti Kebangsaan Malaysia. (12 Nov 2015) |
| Design Machine for Large Scale production | Proposal No. FKP 2014-2, Proposal for DUAL-ANTIBAX machine for large scale production by Faculty of Manufacturing Engineering, Universiti Teknikal Malaysia Melaka (UTeM), Melaka. (09 Oct 2014) |
| Research Grants |
1. Research University Grant (RUG), UTM, Tier 2. Modification of Organo-Clay with Silver and Copper as Antibacterial Agent. 01/05/2012–30/04/2013. RM 31,800.00. Vot. No: 05J05. 2. Prototype Research Grant Scheme (PRGS) from Ministry of Education (MoE) Malaysia. Prototype Development of DUAL-ANTIBAX (Organo-Metal-Clay as Antimicrobial Agent. 01/08/2013–31/07/2015. RM 159,000.00. Vot No: 4L619. |
| Awards |
1. Gold medal in 24th International Invention, Innovation and Technology Exhibition (ITEX) 2013, Kuala Lumpur Convention Centre (KLCC). 9 – 11 May 2013. 2. Bronze medal in Industrial Art & Technology Exhibition (INATEX) 2012, Dewan Sultan Iskandar, UTM. 03 – 05 Oct 2012. 3. Gold Medal in 18th Industrial Art & Technology Exhibition (INATEX) 2016, Dewan Sultan Iskandar, UTM. 04 – 06 Okt 2016. |
| Business Model and Market Analysis | Business proposal: Commercialization of DUAL-ANTIBAX by Innovation and Commercialization Centre (ICC), UTM. (12 Jul 2015) |
PROOF OF CONCEPT
| ITEMS | DOCUMENTATION |
| Theses |
1. Nur Isti’anah Bt Ramli (2012-2015). Organo-Silver-Kaolinite as Antibacterial Agent. MSc (Biosciences). FBME, UTM, Scholarship from MyBrain (MoE). 2. Navitra Muthoovaloo (2014-2015). Characterization and Antibacterial Activity of Hexadecyltrimethylmonium Modified Silver Kaolinite. MSc (Biotechnology) (Mixed Mode, FBME, UTM).
|
| Scientific Articles |
1. Nik Ahmad Nizam Nik Malek, Nur Isti’anah Ramli (2015). Characterization and antibacterial activity of cetylpyridinium bromide (CPB) immobilized on kaolinite with different CPB loadings. Applied Clay Science, 109-110: 8-14. 2. Nik Ahmad Nizam Nik Malek, Siti Amirah Ishak, Mohammed Rafiq Abdul Kadir (2013). Antibacterial activity of copper and CTAB modified clays against Pseudomonas aeruginosa. Advanced Materials Research, 626: 178-182. 3. Nik Ahmad Nizam Nik Malek, Wan Nur Azalisa, Clara Chong Yieh Lin. Antibacterial Activity of Cetyltrimethylammonium Bromide Modified Silver-Bentonite. 6th International Conference on Material and Manufacturing Technology (ICMMT2015), American Society for Research, Bali, Indonesia, 9-11 May 2015. 4. Nor Syafawani Sarah Md Saad, Nik Ahmad Nizam Nik Malek, Chong Chun Shiong, Nur Isti’anah Ramli, Siti Aishah Mohd Hanim, Mashitah Mad Salim, Nur Hidayah Abdullah. Antimicrobial Activity of Copper Kaolinite and Surfactant Modified Copper Kaolinite against Gram positive and Gram negative bacteria. 3rd International Science Postgraduate Conference (ISPC 2015), Ibnu Sina Institute for Fundamental Science Study, UTM, 24-26 Feb 2015. 5. Nur Isti’anah Ramli, Nik Ahmad Nizam Nik Malek, Siti Aishah Mohd Hanim, Mashitah Mad Salim and N. S. Sarah Md Saad. Bactericidal Effect of Kaolinite Modified with Cetylpyridinium Bromide and Silver Ions against Staphylococcus aureus (ATCC 6538). Regional Annual Fundamental Science Symposium 2014 (RAFSS 2014), Persada Johor, Malaysia, 9-10 Sept 2014. 6. Nur Isti’anah Ramli, Mashitah Mad Salim, Siti Aishah Mohd Hanim, Nor Syafawani Sarah Md Saad, Salehhuddin Hamdan, Nik Ahmad Nizam Nik Malek. Zeta Potential Analysis: Effect of CPB-Kaolinite to the Growth of Bacteria. 2nd International Science Postgraduate Conference 2014 (ISPC 2014), Institute Ibnu Sina for Fundamental Science Studies, UTM, 10-12 Mar 2014. |
COMMERCIALIZATION ROUTE/APPROACH
These are the methods to commercialize products under UTM suggested by Innovation and Commercialization Centre (ICC-UTM) and needs to follow the “UTM Intellectual Property Commercialization Policy”.
| OPTION | DESCRIPTION |
|
Spin-off / joint-venture companies
|
· Third parties investments into UTM’s patent to enable the most effective commercialization of the product. · Spin-off type A: UTM has equity and receives up-front payment and royalties · Spin-off type B: UTM will receive only upfront payment and royalties. |
|
Commercialization project
|
· Small commercialization project for production and commercialization of DUAL-ANTIBAX by any interested companies. |
| Licensing | · Manufacturing and marketing DUAL-ANTIBAX by any interested company. In return, UTM and inventor will get a royalty for the selling item. |
ACKNOWLEDGEMENT
- Ministry of Education (MoE) Malaysia
- Makmal Bioserasi, Universiti Kebangsaan Malaysia
- Faculty of Manufacturing Engineering, Universiti Teknikal Malaysia Melaka (UTeM)
- Mineral Research Centre (MRC), Mineral and Geoscience Department Malaysia.
- Universiti Teknologi Malaysia (UTM)
- Faculty of Biosciences and Medical Engineering, UTM
- Centre for Sustainable Nanomaterials (CSNano)
- Nanotechnology Research Alliance, UTM
- Research Management Centre (RMC), UTM
- Innovation and Commercialization Centre (ICC), UTM
- Frontier Materials Research Alliance, UTM
- Institute of Bioproduct Development (IBD), UTM