https://newatlas.com/energy/fast-charging-lithium-battery-five-times-current-safe/

Carefully introducing new materials into the design of today’s lithium-ion batteries has the potential to greatly improve their performance, and scientists have just happened upon a promising possibility in carbon nanotubes. By incorporating these materials into the electrode of a lithium metal battery, the researchers have produced a version that is not only safer, but has the potential to charge up in just a fraction of the time of conventional devices.
The research was carried out at Texas A&M University’s College of Engineering and centers on a battery architecture with huge potential. When a traditional lithium-ion battery charges and discharges, the lithium ions are ferried back and forth between the cathode and anode, the latter of which is usually made from a mix of graphite and copper.
But scientists see a great alternative in using pure lithium metal instead, which offers very high energy density and could make for batteries that charge much faster and offer as much as 10 times the capacity. One study last year described a lithium anode as “critical to break the energy density bottleneck of current li-ion chemistry.” Suffice to say, there is considerable interest in making these things work.
Standing in the way, however, are dangly tentacles called dendrites. These tree-like structures build up on the surface of the anode when lithium ions aren’t evenly deposited and as they grow, they can pierce key components of the battery and cause it to short circuit and or catch fire. If that doesn’t occur, they cause the battery to quickly lose its charge anyway.
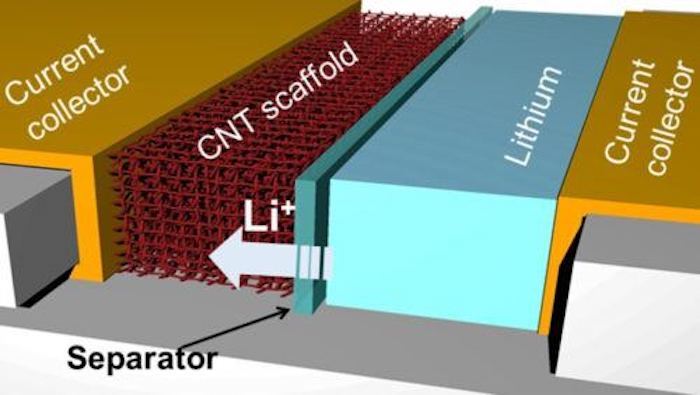
So a great deal of research centers on the problem of dendrite formation, and the Texas A&M team believes it may have found a solution in ultralight and highly conductive carbon nanotubes. The design mirrors that of another experimental battery we looked at in 2018 that uses a thin carbon nanotube film to effectively suffocate dendrites before they properly take shape, but the researchers behind the new study have taken a slightly different approach.
For its anode, the team used carbon nanotubes to build three-dimensional porous scaffolds laced with molecules that cause the lithium ions to bind to its surface. It took some experimentation, but with the right concentration of these binding molecules, the team found it had produced a battery anode that avoided the buildup of dendrites on its surface.
“But when we had just the right amount of these binding molecules, we could ‘unzip’ the carbon nanotube scaffolds at just certain places, allowing lithium ions to come through and bind on to the entire surface of the scaffolds rather than accumulate on the outer surface of the anode and form dendrites,” says study author Juran Noh.
Another consequence of this even and safe distribution of the lithium ions was an increased ability of the battery to produce larger currents. So much so, the team reports that the anode can handle currents five times that of conventional batteries. This raises the prospect of a battery that is not only safer and with greater energy density, but one that can be charged in possibly a fraction of the time.
“Building lithium metal anodes that are safe and have long lifetimes has been a scientific challenge for many decades,” Noh said. “The anodes we have developed overcome these hurdles and are an important, initial step toward commercial applications of lithium metal batteries.”
The research was published in the journal Nano Letters.
Source: Texas A&M