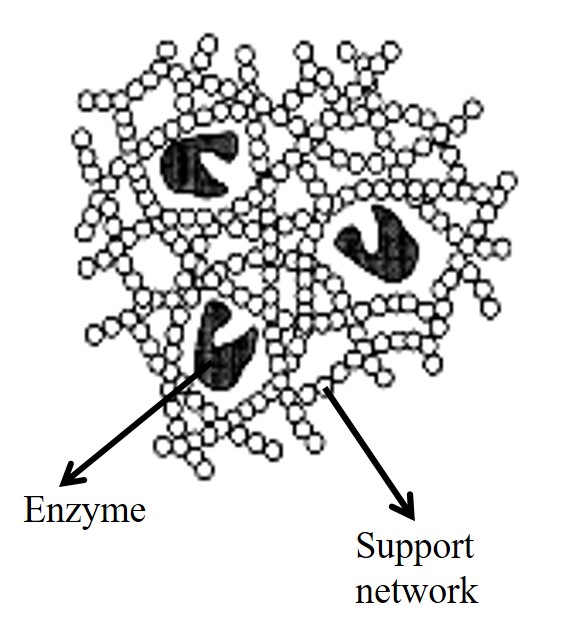
In the process of enhancing the stability and reusability of the enzyme, enzymes are often immobilized by physical or chemical means to the surface of insoluble supports. An immobilized enzyme can be defined as an enzyme which is not freely soluble and whose movement in space is completely or partially restricted to a small region [1]. It is also known as the process of imprisoning an enzyme molecule in a distinct phase that allows for exchange but is separated from the bulk phase in which substrate effectors or inhibitor molecules is dispersed [2]. The immobilized enzyme is usually insoluble in water and the support that is usually used to immobilize enzyme is composed of high molecular weight, hydrophilic polymer. Although its movement is partially or completely restricted within the microenvironment, the term “immobilization” does not imply that the enzyme can never move within its distinct phase [3].
Immobilization of enzymes on solid surface places them in a more natural environment and in many cases; allow them to function more efficiently [2]. When the soluble enzyme is used for catalyzing a reaction, the reaction can only be terminated by deactivating the enzyme or by changing the environment. In the case of the immobilized enzyme, the extent of reaction can be adjusted either by changing the residence time of the reactants or by removing the immobilized enzyme support from the reaction solution [1]. Immobilized enzymes also retain their catalytic properties for a longer period of time, thus making their use even more economical. Another benefit of immobilized enzymes is the inhibition of enzyme activity whereby the excess of the product can be minimized [4]. Immobilized enzymes can also be used for multi-enzyme systems where several enzymes are placed in the same support thus; enabling it to catalyze a sequence reaction [5].
The appropriate matrix or support for the immobilization of enzyme is chosen based on several different properties which affect the production process [80]. One of the properties is that the materials need to have high surface area particularly up to 100 m2/g for high enzyme loadings and high porosity to provide access for the substrate. The immobilization matrix must also be resistant to chemical degradations and mechanical stability. Microbial resistance of matrix is also an important property that needs to be considered since a major concern to any immobilized enzyme process is the presence of microbes. Furthermore, the durability of the carrier is often determined by its resistance to microbial degradation [3].
References:
- Hartmeier, W. Immobilized Biocatalysts: An Introduction. Berlin: Springer-Verlag. 1988.
- Woodley, J. M. New Opportunities for Biocatalysis: Making Pharmaceutical Processes Greener. Trends Biotechnol. 26(6): 321-327.
- Messing, R. A. Immobilized Enzymes for Industrial Reactors. New York: Academic Press Inc. 1975.
- Cheetam, P. S. J. The Applications of Enzymes in Industry: Handbook of Enzyme Biotechnology. London: Ellis. 1995.
- Gerhatz, W. Enzymes in Industry: Production and Applications. Weinheim: VCH Publishers, Inc. 1990.
Source: Nor Suriani Sani (2009). Synthesis and Characterization of Alcohol-Free Tyrosinase Encapsulated Silica Aerogel. MSc (Chemistry), Master Thesis, Universiti Teknologi Malaysia.