Corrosion is a major challenge and essential risk to contain for any oil and gas operator. Loss of containment can mean loss of process, loss of revenue, expensive repairs and – most importantly – a potentially major safety hazard. But while a breach in a cross-country pipeline can be a major incident and environmental risk, it’s another matter downstream. Different plant and processes are tightly packed together, with all manner of hydrocarbons and other combustible fluids boiling, cooling or flowing. Workers dart in-between, never more than a few dozen feet from a potential corrosion risk. In this environment, loss of containment could be catastrophic. It doesn’t take more than a quick google search to find a multitude of incidents related to corrosion control issues.
So how to manage the risk? Most, if not all, refineries and petrochemical plants employ talented and knowledgeable corrosion engineers with the expertise to do so. But to do the best job, they need the best data. That in turn requires a fully integrated monitoring system – something that has historically been in short supply.
An aggressive environment
The difficulty involved in downstream corrosion monitoring can’t be underestimated. And it’s growing.
The refining industry has many elements that can contribute to increased corrosion rates. Corrosive substances can be found in the feedstock – amines, sulphuric acid as an example and often further elements can be added and produced during the refining process itself, such as oxygen, nitrogen, trace metals, salts, carbon dioxide, and naphthenic acids. Refinery processes themselves involve extreme temperatures and velocities in many of the processes including distillation, catalytic crackers, and alkylation that all contribute to elevated corrosion rates.
Not only that, but downstream operators are dealing with more than hydrocarbons. There are a variety of fluids used in different processes, many of which can be extremely corrosive themselves. Getting corrosion right, in this instance, can be the difference between profitability and throwing money away on unscheduled repairs and maintenance.
To add to this the ripple effects of the recent downturn are still being felt across the oil and gas sector. With ageing assets, extended operating windows and high demands on production rates, one technique firms adopted in order to adapt was crude blending, mixing different qualities of conventional and TAN crudes to a level that they hadn’t before. This practice makes financial sense, TAN crudes can be a third of the cost to operators compared to conventional crudes but also make corrosion less predictable and increases risk due to their higher acid content. Prices have recovered, but not to anything like previous highs, and crude blending is still common practice. If refineries are to continue to blend crudes and remain profitable they must ensure that a robust, accurate integrated corrosion monitoring system is in place.
Keeping tabs
In this environment, corrosion is unavoidable. The key is to keep tabs and effectively monitor the issue. And accuracy is key. Underestimate corrosion, and the risk of failure rears its head, along with all the safety and business risks that come with it. However, if an operator errs too much on the side of caution, they risk unnecessary maintenance downtime, premature replacement of equipment or an overzealous corrosion inhibitor programme – all potentially expensive and avoidable mistakes.
But accurate monitoring – across a diverse downstream facility – is easier said than done. Different pipework and different processes require a different approach to corrosion.
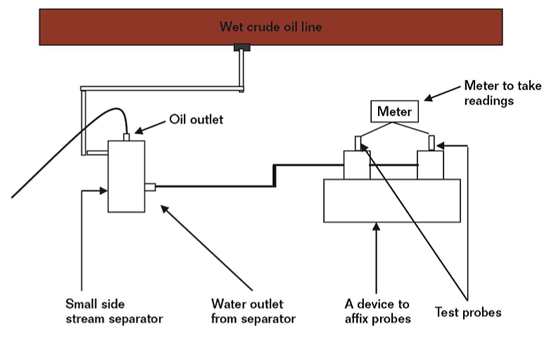
For example, one of the most tried and tested methods for corrosion monitoring is the use of a corrosion coupon. This is a small sample of metal, inserted into the flow at selected locations, which is then subject to the same corrosive factors as the pipework. At regular intervals – perhaps as often as every few months – corrosion coupons are removed and engineers measure how much of the metal has been corroded, taking that as a representation for pipeline corrosion. A corrosion coupon can also give valuable data on the type of corrosion and potential localised pitting corrosion issues. This is appropriate in many instances, but not all.
Another commonly used monitoring method is to insert electrical resistance probes inline into the pipework. These are capable of real-time monitoring and can detect changes in corrosivity within hours, making them ideal for highly changeable applications. High resolution ER probes allow operators to directly monitor levels of corrosion in their system and react quickly to process changes in a system, rather than using a coupon as a proxy. This approach is extremely effective and the only viable method to accurately monitor and control corrosion inhibitor injection programs, as it allows operators to adjust volumes injected based on live, granular data. A huge cost saving potential.
However, as with coupons, there are certain process within the downstream industry where intrusive monitoring cannot be used due to extreme process conditions. It isn’t appropriate for all applications.
That’s why, in many cases, corrosion engineers have turned to external ultrasound thickness (UT) monitoring systems that affix directly to the outside of the pipe and require neither downtime nor intrusion to install. However, by itself the approach is no silver bullet. The trade-off for these advantages is that operators have to settle for a lower level of sensitivity, resolution and accuracy. Modern ultrasonic technology has made great progress on this front, but still doesn’t match ER probes for accuracy and response times.
So, there’s no one-size-fits-all perfect solution for corrosion engineers working downstream. Likely they will have a patchwork of different systems, selecting the best option process by process, pipe by pipe. This gives the best possible corrosion monitoring performance at the individual application level, but carries its own risks at the facility-wide scale.
The holy grail for the corrosion engineering team is an overall view of corrosion risk across the facility. Understanding which equipment is suffering from near problematic corrosion levels, and which other processes are in close proximity, helps give a more accurate gauge of overall risk to the operator and personnel. Similarly, understanding if one process is due downtime for corrective maintenance helps operators plan more effectively. For example, if one process has knock-on effects on another, it may best to schedule maintenance for both at the same time even if one isn’t quite at its corrosion limits, rather than have to shut down a second time a couple of months down the line.
The only way for corrosion engineers and operators to effectively monitor plant wide is through assimilating those disparate systems into one broader integrated system, incorporating corrosion coupon, ER probe and UT devices together feeding the data back into a central platform to give a holistic, facility-wide view of risk. Corrosion engineers are then empowered to make the most informed decisions, guarding safety while maximising asset profitability. In the past, this might have been a pipe dream. However, companies like Cosasco now have decades of accumulated experience with these individual technologies and have invested in platforms to bring them together in a fully integrated way.
So, for corrosion engineers at refineries and petrochemical plants, there’s really no excuse not to implement an integrated, multi-pronged corrosion monitoring strategy. No excuse because the risks to safety and revenue are too high to ignore, and no excuse because the technological limitations that may have once hampered such a programme are no longer insurmountable. A modern system utilises intrusive electrical resistance probes and state of the art, high accuracy non-intrusive ultrasonic ones. Feeding that data back into a central view of risk, is the logical next step in keeping the downstream sector safe and profitable.
 Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International and other venues.
Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International and other venues.
 Aluminum oxide.
Aluminum oxide.
 Structures such as offshore drilling platforms supported by steel piles may be protected with either sacrificial galvanic anode systems or impressed current systems.
Structures such as offshore drilling platforms supported by steel piles may be protected with either sacrificial galvanic anode systems or impressed current systems.
 Reinforced concrete structures, such as bridges, can be exposed to aggressive chloride environments and often show evidence of corrosion after short service periods.
Reinforced concrete structures, such as bridges, can be exposed to aggressive chloride environments and often show evidence of corrosion after short service periods.










 Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International. This year ihe is presenting on
Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International. This year ihe is presenting on