Cathodic Protection: Industrial Solutions for Protecting Against Corrosion
By Volkan Cicek (360 pages)
Price USD 175 (RM 750 – This book is available in the PSZ library TA418.74 C53)
A companion to the title Corrosion Chemistry, this volume covers both the theoretical aspects of cathodic protection and the practical applications of the technology, including the most cutting-edge process and theories. Engineers and scientists across a wide range of disciplines and industries will find this the most-up-to-date, comprehensive treatment of cathodic protection available. A superb reference and refresher on the chemistry and uses of the technology for engineers in the field, the book also provides a tremendous introduction to the science for new comers to the field

 Mehrooz Zamanzadeh conducts a below-grade corrosion assessment of a buried tower leg. Photo courtesy of Mehrooz Zamanzadeh and BC Hydro Transmission.
Mehrooz Zamanzadeh conducts a below-grade corrosion assessment of a buried tower leg. Photo courtesy of Mehrooz Zamanzadeh and BC Hydro Transmission.







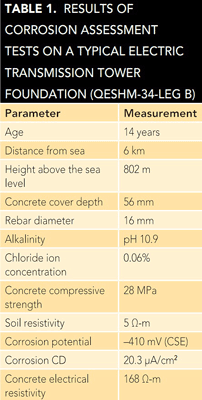 processed by software developed in-house based on an artificial neural network. This software classified the examined tower foundations into one of four corrosion risk categories (low, medium, high, and very high). The results showed ~60% of the selected foundations were placed in either the high or very high corrosion risk groups.
processed by software developed in-house based on an artificial neural network. This software classified the examined tower foundations into one of four corrosion risk categories (low, medium, high, and very high). The results showed ~60% of the selected foundations were placed in either the high or very high corrosion risk groups.