Corrosion Basics – An Introduction, Second Edition
By Pierre Roberge (364 pages)
Price: USD 215 (RM 924.50) Opps! almost RM1K for this book, fret not as staff and student in UTM Skudai Johor who interested to have some basic knowledge in corrosion you can check out the book in PSZ library – TA418.74 R634
Also rank as top 10 bestseller for corrosion book!
This second edition is an extensive update to the fist and represents NACE’s all-time best selling book. The book presents basic concept to understand how corrosion problems occur and how they can be prevented or controlled with modern tools. It discusses environments, materials and how they corrode, methods of corrosion control, and corrosion testing and monitoring.


 A worker cleans the surface of a column in a ship’s tank.
A worker cleans the surface of a column in a ship’s tank.
 An aging pipeline is being prepared for recoating. Photo courtesy of Jeffrey Didas.
An aging pipeline is being prepared for recoating. Photo courtesy of Jeffrey Didas.




 High-temperature corrosion is a widespread problem at facilities in various industries, including refineries and petrochemical plants.
High-temperature corrosion is a widespread problem at facilities in various industries, including refineries and petrochemical plants.









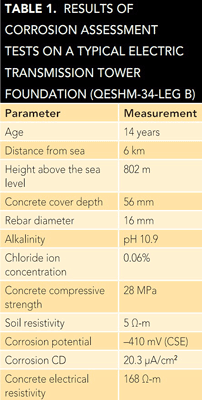 processed by software developed in-house based on an artificial neural network. This software classified the examined tower foundations into one of four corrosion risk categories (low, medium, high, and very high). The results showed ~60% of the selected foundations were placed in either the high or very high corrosion risk groups.
processed by software developed in-house based on an artificial neural network. This software classified the examined tower foundations into one of four corrosion risk categories (low, medium, high, and very high). The results showed ~60% of the selected foundations were placed in either the high or very high corrosion risk groups.