 By Materials Performance on 6/29/2017 3:25 PM
By Materials Performance on 6/29/2017 3:25 PM
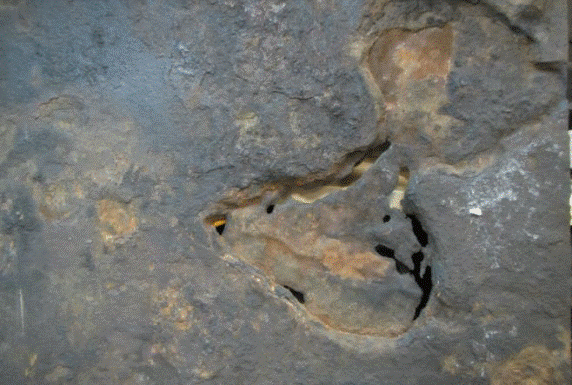
Soil side of the failed plate sample.
Arefinery was experiencing bottom plate underside (soil-side) corrosion of its aboveground storage tanks (ASTs) at an extraordinarily high rate of 1 to 2 mm/y, which resulted in the failure of four tanks within a two-year period that occurred seven years after the refinery was commissioned. All four tanks had severely damaged bottom plates, which were constructed of 8-mm thick uncoated carbon steel.
Underside corrosion protection for the ASTs was provided by an impressed current cathodic protection (ICCP) system using a mixed-metal oxide (MMO) grid anode system. The corrosion morphology after the failure revealed severe localized corrosion with large, deep pits found under deposits of orange-red tubercles comprised of iron oxides.
In CORROSION 2017 paper no. 9025, “Premature Failure of API 650 Oil Storage Tank Bottom Plates Due to Soil Side Corrosion,” authors N. Al Abri, J.R Nair, A. Al Ghafri, and F. Al Mawali describe the failure analysis carried out, which included a review of the design and function of the CP system and metallurgical tests of a failed bottom plate sample.

For the ICCP system, the MMO anode grid was placed between a high-density polyethylene (HDPE) secondary containment liner and the tank bottom, which sat on a 75-mm thick sand pad. The underside of the bottom plates was uncoated and the CP design was based on 100% bare surface area.
The failures occurred between July 2013 and August 2015, and a detailed CP survey was carried out for the storage tanks in February 2016. The results revealed that none of the tanks achieved the NACE SP0193-20161 protection criterion of –850 mV instant “off” potential. The potentials varied from –200 to –800 mV vs. a copper/copper sulfate (Cu/CuSO4) electrode (CSE), with an average “off” potential of –450 mV vs. CSE across all tanks.
Since the potentials were not meeting the –850 mV criteria, further investigation was done to understand the possible reasons for such low polarized potentials. The authors note that design deficiencies of the ICCP system—primarily the anode depth/spacing and inappropriate distribution of power feed cables—resulted in a nonuniform potential profile across the tank bottom surface. High current and voltage attenuation along the anode grid did not provide sufficient current and polarization at a distance away from the power feeds, which led to under-protection of the tank bottom.
Because an inefficient CP system alone typically doesn’t cause such aggravated corrosion, a sample from the failed tank bottom was sent to an external laboratory for a detailed metallurgical analysis to identify corrosion products and possible corrosion mechanisms. A form of underdeposit corrosion (UDC) that can corrode steel at the rate of 1 mm/year is microbiologically influenced corrosion (MIC).

X-ray diffraction analysis of the sample plates’ soil side indicated the tubercles were made of porous layers or strata consisting mainly of iron oxides surrounded by magnetite (Fe3O4). The scales appeared to consist of multiple layers. A closer analysis performed on flakes from scale on the pits indicated the probability of iron-oxidizing bacteria (IOB) present in the corrosion, which formed deposits that further aggravated the corrosion by forming a differential aeration cell. IOB produce orange-red tubercles of iron oxides and hydroxides by oxidizing ferrous ions from the bulk medium or the substratum.2
The corrosion rates observed were amplified by the ingress of water, primarily from leaking fire water sprinklers, through gaps between the annular plate and foundation, which brought in bacteria and corrosive anions such as chlorides and sulfates. Without an effective CP system and no other means of corrosion control, the tank floor was exposed to a severe form of bacterial and UDC that led to perforation and loss of inventory.
References
1 NACE SP0193-2016 (formerly RP0193-2001), “External Cathodic Protection of On-Grade Carbon Steel Storage Tank Bottoms” (Houston, TX: NACE, 2016).
2 A.W. Peabody, Peabody’s Control of Pipeline Corrosion, 2nd ed., R. Bianchetti, ed. (Houston, TX: NACE International, 2001), p. 279-280.

Recent Comments