
 Aluminum oxide.
Aluminum oxide.
The thin coating layer is expected to be especially useful for preventing the permeation of tiny molecules, such as hydrogen gas or radioactive tritium (a heavy form of hydrogen that forms inside the cores of nuclear power plants), that can pass through most materials.
Most metals, with the notable exception of gold, tend to oxidize when exposed to air and water. This reaction, which produces rust on iron, tarnish on silver, and verdigris on copper or brass, can weaken the metal over time and lead to cracks or structural failure. There are three known elements, however, that produce an oxide that can actually serve as a protective barrier and prevent further oxidation: aluminum oxide (Al2O3), chromium oxide (Cr2O3), and silicon dioxide (SiO2).
“We were trying to understand why aluminum oxide and silicon dioxide are special oxides that give excellent corrosion resistance,” says Ju Li, a professor of nuclear engineering and science at MIT and senior author of a paper describing the new finding.
The team, led by MIT graduate student Yang Yang, used highly specialized instruments to observe in detail the surface of metals coated with these special oxides to see what happens when they are exposed to an oxygen environment and placed under stress. While most transmission electron microscopes (TEMs) require samples to be studied in a high vacuum, the team used a modified version called an environmental TEM (E-TEM), which allowed the samples to be studied in the presence of gases or liquids of interest. The device was used to study the process that can lead to stress corrosion cracking.
Metals under stress from pressure inside a nuclear reactor vessel and exposed to an environment of superheated steam can corrode quickly if not protected. Even with a solid protective layer, cracks can form that allow oxygen to reach the bare metal surface, where it can then penetrate interfaces between the metal grains that make up a bulk metal material, and cause further corrosion that infiltrates even deeper and leads to structural failure. “We want an oxide that is liquid-like and crack-resistant,” Yang says.
It turns out that aluminum oxide can have liquid-like flowing behavior, even at room temperature, if the coating layer is thin enough—about 2- to 3-nm thick.
People typically think that the metal oxide would be brittle and subject to cracking, Yang says, explaining that no one had demonstrated otherwise because it is so difficult to observe the material’s behavior under realistic conditions. That’s when the specialized E-TEM setup at Brookhaven National Laboratory (Upton, New York, USA), one of about 10 such devices available in the world, came into play. “No one had ever observed how it deforms at room temperature,” he adds.
“For the first time, we’ve observed this at nearly atomic resolution,” notes Li. This approach demonstrated that an Al2O3 layer, normally so brittle it would shatter under stress, is almost as deformable when made exceedingly thin as a comparably thin layer of aluminum metal (much thinner than aluminum foil). When a bulk piece of aluminum is coated with Al2O3, the liquid-like flow keeps the aluminum covered with its protective layer, Li reports.
The researchers demonstrated inside the E-TEM that the aluminum with its Al2O3 coating could be stretched to more than double its length without causing any cracks to form, Li says. The oxide forms a very uniform conformal layer that protects the surface, with no grain boundaries or cracks, even under the strain of stretching, he says. Technically, the material is a type of glass, but it moves like a liquid and fully coats the surface as long as it is thin enough.
The self-healing coating could have many potential applications, Li says, noting the advantage of its smooth, continuous surface without cracks or grain boundaries.
Source: Massachusetts Institute of Technology, news.mit.edu.








 Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International. This year ihe is presenting on
Gregory Havel is a member of the Town of Burlington (WI) Fire Department; retired deputy chief and training officer; and a 35-year veteran of the fire service. He is a Wisconsin-certified fire instructor II, fire officer II, and fire inspector; an adjunct instructor in fire service programs at Gateway Technical College; and safety director for Scherrer Construction Co., Inc. Havel has a bachelor’s degree from St. Norbert College; has more than 35 years of experience in facilities management and building construction; and has presented classes at FDIC International. This year ihe is presenting on 
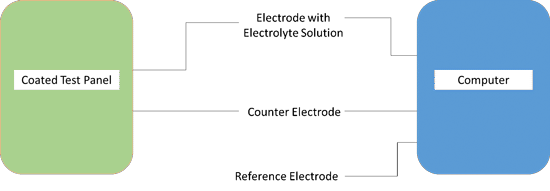
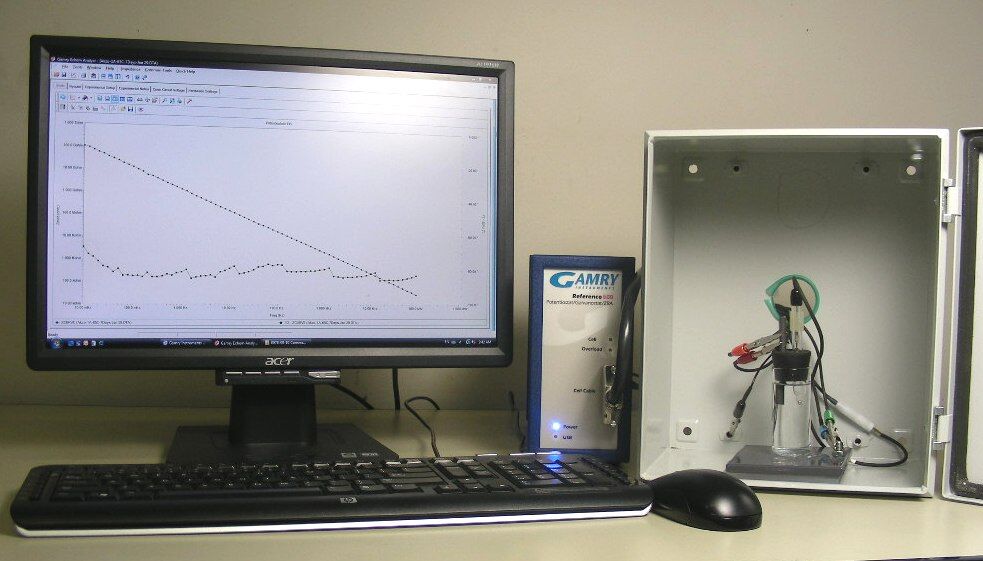 EIS testing apparatus. Photo courtesy of Amal Al-Borno, Charter Coating Service (2000), Ltd.
EIS testing apparatus. Photo courtesy of Amal Al-Borno, Charter Coating Service (2000), Ltd.
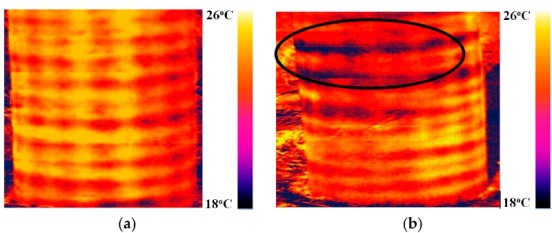
 The study was completed at Russia’s Trans-Siberian Railway, using an experimental facility for conducting induction and infrared thermal imaging. Photo courtesy of TPU.
The study was completed at Russia’s Trans-Siberian Railway, using an experimental facility for conducting induction and infrared thermal imaging. Photo courtesy of TPU.
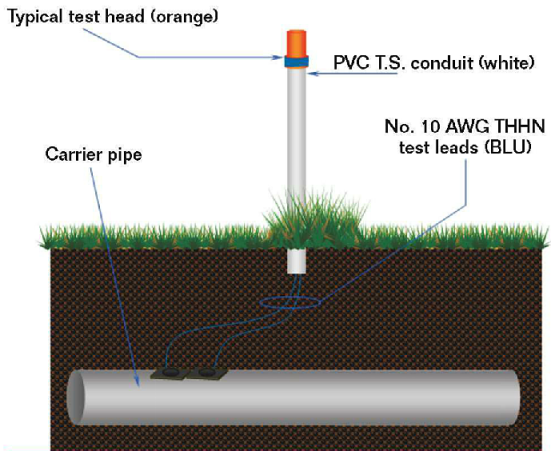
 During the installation of a new pipeline, corrosion engineers must consider many different aspects of the pipeline and its corrosion protection.
During the installation of a new pipeline, corrosion engineers must consider many different aspects of the pipeline and its corrosion protection.



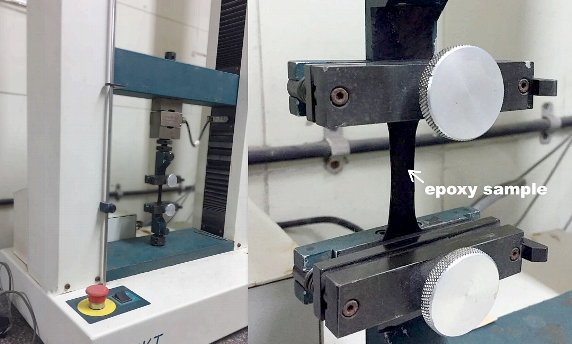 Tests were conducted on the chemical and mechanical properties of the graphene-added epoxy coatings, including the tensile strength. Photo courtesy of Talga Resources.
Tests were conducted on the chemical and mechanical properties of the graphene-added epoxy coatings, including the tensile strength. Photo courtesy of Talga Resources.





