Taken from – http://www.european-coatings.com/Raw-materials-technologies/Applications/Protective-Marine-coatings/Anticorrosion-properties-of-epoxy-nanochitosan-nanocomposite-coatings
Tuesday, 24 October 2017
Nanochitosan was prepared to act as a nanofiller reinforcing agent that had been incorporated in a protective coating for corrosion protection using high-molecular-weight chitosan.
Characterisation methods
The surface morphology and structural characterisation of the NCH and nanocomposite coatings were carried out using Field Emission Scanning Electron Microscope (FESEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy. Optical characterisation of the nanocomposite specimens was examined by UV-vis spectroscopy at a range of 300-800 nm in transmission mode. The thermal analysis was employed using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The corrosion protection performances of the nanocomposite coated mild steel substrates were comparatively studied using electrochemical impedance spectroscopy (EIS).
Enhanced anticorrosion properties
The results showed that all the epoxy resin based nanocomposite coating containing NCH significantly increases the anticorrosion properties. The incorporation of 0.5% nanochitosan exhibited the overall best performance.
The study is published in: Progress in Organic Coatings, Volume 113, December 2017


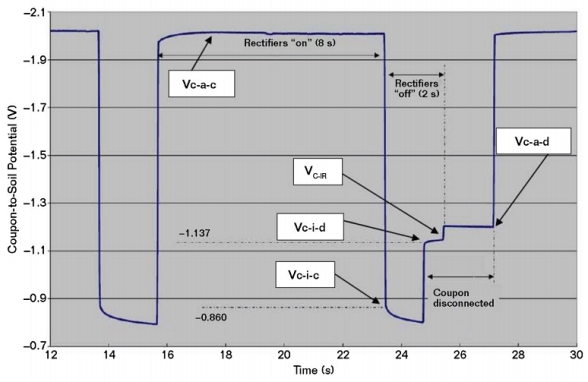
 Coupons can be used to assist in the evaluation of CP levels in buried steel piping.
Coupons can be used to assist in the evaluation of CP levels in buried steel piping.
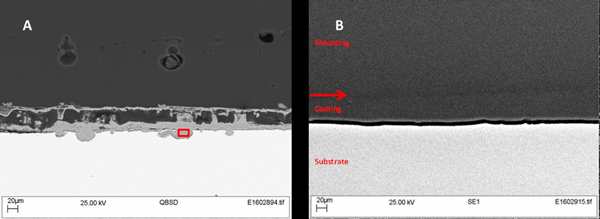
.jpg)





 FIGURE 1: Epoxy-coated steel panels before (A, B, C) and after (D, E, F) 1,000 h of salt fog testing: (A) 0% graphene control at 0 h; (B) 0.5% Sample 2 at 0 h (C) 5.0% Sample 1 at 0 h; (D) 0% graphene control at 1,000 h; € 0.5% Sample 2 at 1,000 h; and (F) 5.0% Sample 1 at 1,000 h. Photos courtesy of AGM.
FIGURE 1: Epoxy-coated steel panels before (A, B, C) and after (D, E, F) 1,000 h of salt fog testing: (A) 0% graphene control at 0 h; (B) 0.5% Sample 2 at 0 h (C) 5.0% Sample 1 at 0 h; (D) 0% graphene control at 1,000 h; € 0.5% Sample 2 at 1,000 h; and (F) 5.0% Sample 1 at 1,000 h. Photos courtesy of AGM.