Written by Matt Jones and Jim Sokolowski, Tessella Thursday, 01 June 2017 00:00
Matt Jones and Jim Sokolowski, of Tessella, show how data analytics can give insight into corrosion rates to reduce risk and maintenance costs.
 |
| Riser and slip joint on a semisubmersible oil rig. Photo from iStock. |
Corrosion is a fact of life in offshore environments, and offshore production costs are significantly impacted by it. It is often a known unknown, and this makes business and investment decisions that need to consider its impact harder and riskier.
This offers an even trickier proposition when facilities are nearing the end of their life and alternatives, such as carbon capture and storage (CCS) in depleted reservoirs, are being considered.
Such an alternative was being considered by an operator in the North Sea. But, the firm wanted to understand the possible causes of corrosion on its existing facility, to be able to predict future corrosion accurately. These insights could be used to make other decisions, such as how to optimally plan expensive corrosion re-measurement campaigns.
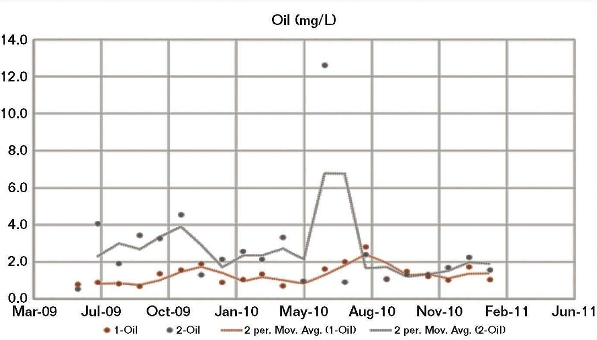
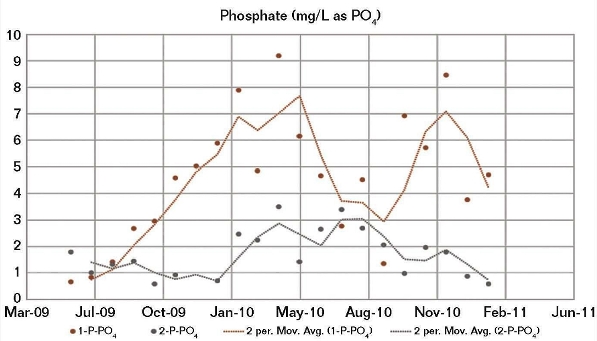
The company had just less than 10 years of historical eddy current data about surface casing and conductor corrosion from scheduled measurement campaigns, as well as original spud data. However, there was a need to truly understand what this data meant. The data showed that some wells had significantly worse corrosion than others that appeared to be “similar,” but they were unsure why.
As mentioned, some of the historical data was eddy current measurements – a common corrosion test by which a magnetic field is applied to the material, and variations in the electrical conductivity and magnetic permeability are used to map corrosion – and there were significant questions about the validity of these measurements. Taking accurate corrosion measurements like these are complex and often the uncertainties and errors can be so large that the measurement ranges from useful, to useless.
Tessella started a first principles analysis to deepen the understanding of the root cause of corrosion by exploring 20GB of data acquired from historical operational measurements from the PI historian, captured over nearly 20 years. The data did not require special processing or cleaning prior to use.
Our data scientists explored the time histories of all the operational data for temperatures, pressures, production rates and water rates, and used a range of statistical techniques (principal component analysis, dimensionality reduction and cluster identification) to find structure in this history. They examined whether any of these clusters correlated with the corrosion levels, then drilled down into the underlying variables to understand what element in the historical record was driving this.
By applying knowledge of corrosion chemistries and environmental mechanisms that accelerate the corrosion process, the operational details from the historic data could be used to determine possible likely causes of the high corrosion in the affected casing.
Next, Tessella’s data scientists focused on estimating the future corrosion of casings in a new study in a depleted field, to help assess its potential long-term use in a CCS capacity.
This work had started from a scientific publication in 2005 that had presented a methodology to estimate corrosion rates more effectively. The approach assumes a particular stochastic corrosion model, and then uses Bayesian probability techniques to estimate the associated parameters, such as mean corrosion rate.
The analytics team then enhanced this Bayesian approach to be more appropriate to the issue of interest. This required analyzing and modeling the errors in the corrosion measurement process, developing a new understanding, as well as leveraging prior information using data from previous fields.
The resulting models were able to predict mean corrosion rates of well casings across the second depleted field, as well as associated uncertainties and also sensitivities of the results to the various assumptions made. This allowed our data scientists to understand how far existing infrastructure has corroded, and predict the associated future lifetime, and hence suitability, for use in the CCS context.
In addition, the analysis showed the ability to optimize future corrosion measurement campaigns based on individual well corrosion predictions and uncertainties, with associated cost savings.
The key to successful data projects
There are several factors that made this data project a success. First, there was a clear objective and business question. The approach was then focused on using the data to look for the insight needed.
Second, those involved understood the business, scientific and engineering challenge, as well as the data. Meaning, the context of what the data was telling them was understood, and they could hypothesize about what correlations mean, and then rigorously test them to establish causation.
Finally, the project looked at how data could quickly address a specific problem and in a timescale of weeks, not years. By taking the right approach, data analytics can deliver real business value, quickly.






 By Ali Morshed on 6/1/2017 11:57 AM
By Ali Morshed on 6/1/2017 11:57 AM Improvement in the optimization of corrosion costs can boost the financial bottom line for many oil and gas assets.
Improvement in the optimization of corrosion costs can boost the financial bottom line for many oil and gas assets.