By Kathy Riggs Larsen on 10/31/2016 3:33 PM

Carbon capture and storage (CCS) is a carbon sequestration method that minimizes the release of carbon dioxide (CO2) into the atmosphere when burning carbon-based fuels. Primarily, CCS involves capturing the CO2emissions from fossil fuels used in electricity generation and industrial processes, separating it from some other gases if needed, compressing it, and then transporting and injecting it into a storage site such as depleted oil and gas wells or saline aquifers to ensure long-term isolation from the atmosphere. According to the Carbon Capture and Storage Association, CCS technology can capture up to 90% of CO2 emissions.1
Before it will be widely adopted by industry, CCS needs to be proved to be economically viable; however, corrosion issues can arise when separating, transporting, and storing high-pressure, wet CO2, says NACE International member Shiladitya Paul with TWI (Cambridge, United Kingdom). Although the CCS concept is based on a combination of known technologies, he says, large-scale adoption and integration of existing individual technologies poses challenges. Paul notes that corrosion concerns can be different depending on the process stage, and understanding these issues, filling in any technology gaps, and mitigating the corrosion is important for full-scale implementation of CCS as a CO2 emissions reduction tool.
Low-alloy steel and carbon steel (CS) tend to corrode in the presence of wet CO2 due to the formation of carbonic acid (H2CO3). The presence of combustion constituents such as sulfur oxides (SOx), nitrogen oxides (NOx), and other contaminants in the CO2 stream, along with chemicals such as amines used in the CO2 capture process, can form acidic solutions when in contact with water that are corrosive to a variety of metals and alloy systems. In situations where wet hydrogen sulfide (H2S) is present, cracking can be an issue for high-strength steels. If the corrosion rate is too high in these environments, corrosion-resistant alloys (CRAs) may be used. The cost of these materials, though, can be prohibitive.
In his CORROSION 2015 paper no. 5939, “Thermally Sprayed Corrosion Resistant Alloy Coatings on Carbon Steel for Use in Supercritical CO2 Environments,”2 Paul discusses an experiment where the corrosion resistance of CS samples thermally sprayed with various CRA coatings was tested in an environment that recreated conditions that may be found during CO2 transportation and storage. The concept, he explains, combines the corrosion resistance of a CRA coating with the structural integrity of CS as an economical alternative to structural components fabricated of a monolithic CRA for CCS and other applications where supercritical CO2 (CO2 held in a fluid state at or above its critical temperature and pressure) is encountered. If the CRA coating is damaged, it can be repaired and the component reused without costly replacement. Although metals, alloys, and welds have been tested and characterized in low-pressure CO2 and CO2/H2S systems for a number of years, information on their use under higher pressures is limited and test data are needed for materials to be used with confidence in high-pressure applications.
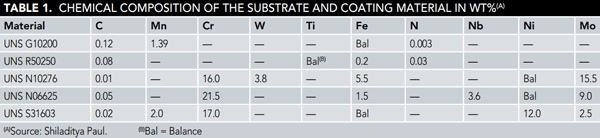
For the experiment, four CRAs were selected—UNS N10276, UNS N06625, UNS S31603, and UNS R50250—that have a proven track record for corrosion resistance in environments similar to the CCS environment or harsher, and are also available in powder form. Generally, CRA refers to a metal that can withstand corrosion in a given environment, Paul says. Three of these CRAs have ≥16% chromium, which enables them to form a thin, tenacious, corrosion-resistant chromium oxide surface layer that protects the substrate from the harsh CO2 and H2S environment. The fourth CRA (UNS R50250) is comprised mainly of titanium, which is known to perform well in corrosive environments. The composition of the metals used is given in Table 1. These metals were thermally sprayed onto the surface of 5-mm thick, 40 by 40 mm square coupons cut from CS (UNS G10200) plate. The metal coating was applied using a high-velocity oxy-fuel (HVOF) thermal spray process. The coatings were nominally 300-µm thick.
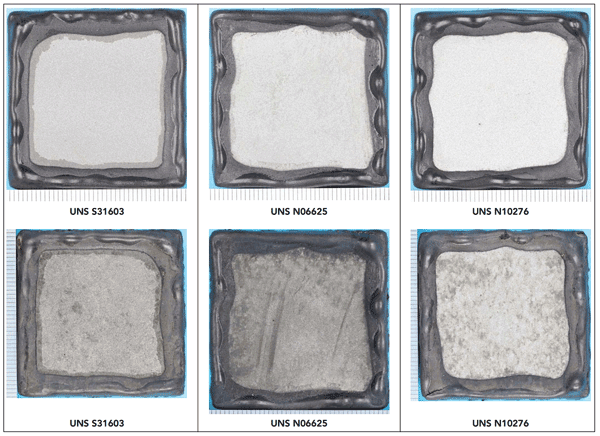
The CRA-coated test coupons and an uncoated CS control coupon were exposed for 30 days in an autoclave with a 3.5 wt% sodium chloride (NaCl) solution. The autoclave was heated to 40 °C and the test pressure was raised to 10 MPa by pumping in a mixture of 95% CO2 and 5% H2S. The test was carried out in a deaerated environment to avoid elemental sulfur and oxide rust. For this experiment, the researchers were observing the combined effect of CO2 and H2S under the very high pressure that would be experienced in CCS applications, along with the aggressive environment. “I would expect that if the CRA could survive this environment, it is likely to function fairly well in other CCS environments that one might see; however, one needs to test in the environment envisaged,” comments Paul. After exposure, the specimens were taken out, air dried, and photographed. Photographs of Cr alloy-coated specimens before and after testing are shown in Figure 1.
A cross-sectional examination was performed and the coupons’ microstructure evaluated to see if the CRA coatings protected the underlying substrate. Microstructural characterization revealed that the bare steel formed a mackinawite (iron sulfide) scale and corroded at a rate of ~0.3 mm/y, while the thermal spray coating layers protected the steel substrate from CO2/H2S corrosion. In all cases, none of the CRA coatings experienced corrosion of the CRA material itself. When sectioned and viewed under a microscope, some porosity was seen in these coatings, which is expected in thermal spray coatings, but very little evidence of corrosion was seen at the coating-substrate interface. Only the titanium coating (UNS R50250) showed some evidence of corrosion at the interface. From micrographs taken of the cross section, it was apparent that the titanium coating had more intersplat porosity than the other coatings, which could explain the formation of some corrosion product at the coating-substrate interface.
Paul explains that in a thermal spray coating process, the consumable (metal powder, wire, or rod) is heated to a molten state. The molten particles are then accelerated toward the component being coated; and when each molten particle hits the substrate at a high velocity, it forms a flat, pancake-shaped deposit called a “splat.” The splats overlap to create a lamellar structure, and intersplat porosity refers to spaces or voids that form between the splats. Some of these pores can be connected and form a pathway for contaminants to reach the substrate and initiate corrosion.
On the titanium-coated sample, the corrosion products were only present at certain locations where through-thickness porosity was present. “What we think happened is the environment—the sodium chloride solution saturated with 95 percent carbon dioxide and 5 percent hydrogen sulfide—entered into the pores and reached the carbon steel, where it started corroding the substrate,” says Paul. Although the titanium material itself is very corrosion resistant, he notes, the barrier performance of the coating depends on the quality of the coating provided by the material.
While it is virtually impossible to achieve a thermal spray coating that is completely free of porosity, he adds, the parameters of the thermal spray process (such as the particle size, spray distance, powder flow rate, etc.) can be optimized to obtain a denser coating that would perform better than the one in the study. He mentions one example of “cold spray,” where particles are heated instead of melted and high velocities deform the particles as they impact the substrate. With careful selection of spray parameters, this can result in a dense coating with almost no through-thickness porosity.
The researchers concluded that HVOF-sprayed coatings comprised of UNS R50250, UNS N10276, UNS N06625, and UNS S31603 can provide a cost-effective corrosion mitigation method for infrastructure likely to be in contact with a mixture of wet supercritical CO2and H2S. The same coatings can possibly be used as an inner lining of pipes for transport of impure CO2. However, Paul notes, care must be taken to ensure that the thermal spray layer does not have any through-thickness porosity or the coating may accelerate corrosion of the underlying steel due to galvanic interactions. If through-thickness porosity is present, then sealants that are resistant to supercritical CO2, H2S, and H2CO3should be used to fill the pores so the coating system is capable of providing a cost-effective corrosion mitigation solution.
Editor’s note: In paper no. 7669, “Performance of Thermally Sprayed Corrosion Resistant Alloy Coatings on Carbon Steel in Supercritical CO2 Environments,” presented at CORROSION 2016 in Vancouver, British Columbia, Canada, Paul reports on the likelihood of accelerated corrosion of the underlying CS when the CRA coating is damaged. In a separate experiment that expands on this work, 8-mm holes (holidays) were drilled through the coatings to expose the underlying steel. After a 30-day exposure to a 3.5 wt% NaCl solution, the specimens were examined with scanning electron microscopy coupled with energy dispersive x-ray spectroscopy (SEM/EDX).
Contact Shiladitya Paul, TWI—e-mail: shiladitya.paul@twi.co.uk.
References
1 “What is CCS?” The Carbon Capture and Storage Association, http://www.ccsassociation.org/what-is-ccs/ (April 4, 2016).
2 S. Paul, “Thermally Sprayed Corrosion Resistant Alloy Coatings on Carbon Steel for Use in Supercritical CO2 Environments,” CORROSION 2015 paper no. 5939 (Houston, TX: NACE International, 2015).